
Your seamless portal to your patient's testing result from NuvoAir Spirometer.
The NuvoAir Digital Therapeutic Platform

Air Next Spirometer
Designed and manufectured by Nuvo Air AB, Air Next spirometer, a class IIa medical device, which measures the same parameters as a GOLD standard spirometer. CE and FDA approved.
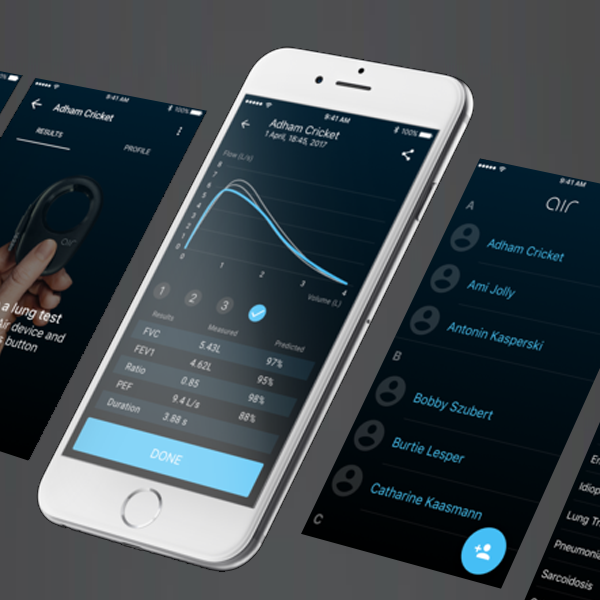
AirMD Mobile Application
The user friendly and beautifully designed mobile application enables user to exercise correct maneuver in constructing accurate lung function tests at professional standard.

Air MD Connect Platform
Professionally customized web platform creates solid bridging between testing results with clinical application. Professional report is approved by multiple HP’s patient record committees.
Proven Result in NuvoAir Solution

NPS 75
solid recognition from users
>95%
retention on NuvoAir solution
39.9%
reduction of urgent consultationsWorld Leading Product with Localized Service

NuvoAir AB
Headquartered in Stockholm, Sweden, NuvoAir is a digital therapeutics company focused on enabling the right clinical decisions surrounding respiratory health in order to improve clinical outcomes. The Air Next Spirometer and AirMD is NuvoAir's top solution in respiratory disease care.

Eir Innotech
Innovated various digital therapeutical solutions, Eir Innotech is professional medical device & solution development and distribution company. Beyond proprietary technology in MDI-based compliance sensor, Eir Innotech is the authorized distributor for NuvoAir in Taiwan area .

BalDr Strategic Consulting
BalDr is a leading pharmaceutical consultancy company in Taiwan, and focuses in various approaches to extract valuable insights from data – from constructing data flow, formulate value creation model, and to onsite deployments. BalDr is the long-term partner of Eir Innotech in solution design and delivery.
Want to Learn More?
Please contact our dedicate expert for more information at service@nuvoair.asthmahelps.com